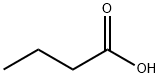
Butyric acid: Production, applications and pharmacodynamics
General description
Butyric acid is a straight-chain alkyl carboxylic acid and its isomer is isobutyric acid (2-methyl propanoic acid). Salts and esters of butyric acid are known as butyrates or butanoates. The acid does not occur widely in nature, but its esters are widespread. It is a common industrial chemical and an important component in the mammalian gut. Butyric acid was first observed in impure form in 1814 by the French chemist Michel Eugène Chevreul. By 1818, he had purified it sufficiently to characterize it. Triglycerides of butyric acid compose 3–4% of butter. When butter goes rancid, butyric acid is liberated from the glyceride by hydrolysis. It is one of the fatty acid subgroups called short-chain fatty acids. Butyric acid is a typical carboxylic acid that reacts with bases and affects many metals. It is found in animal fat and plant oils, bovine milk, breast milk, butter, parmesan cheese, body odor, vomit, and as a product of anaerobic fermentation (including in the colon). It has taste somewhat like butter and an unpleasant odor. Mammals with good scent detection abilities, such as dogs, can detect it at 10 parts per billion, whereas humans can detect it only in concentrations above 10 parts per million. In food manufacturing, it is used as a flavoring agent. Its appearance is as follows:

Figure 1 Appearance of Butyric acid
Production
In industry, butyric acid is produced by hydroformylation from propene and syngas, forming butyraldehyde, which is oxidized to the final product.[1] It can be separated from aqueous solutions by saturation with salts such as calcium chloride. The calcium salt, Ca(C4H7O2)2·H2O, is less soluble in hot water than in cold.
Applications
Butyric acid is used in the preparation of various butyrate esters. It is used to produce cellulose acetate butyrate (CAB), which is used in a wide variety of tools, paints, and coatings, and is more resistant to degradation than cellulose acetate. However, CAB can degrade with exposure to heat and moisture, releasing butyric acid.[2] Low-molecular-weight esters of butyric acids, such as methyl butyrate, have mostly pleasant aromas or tastes. As a consequence, they are used as food and perfume additives. It is an approved food flavoring in the EU FLAVIS database (number 08.005). Due to its powerful odor, it has also been used as a fishing bait additive. Many of the commercially available flavors used in carp (Cyprinus carpio) baits use butyric acid as their ester base; however, it is not clear whether fish are attracted by the butyric acid itself or the substances added to it. Butyric acid was, however, one of the few organic acids shown to be palatable for both tench and bitterling.[3] The substance has also been used as a stink bomb by the Sea Shepherd Conservation Society to disrupt Japanese whaling crews.
Pharmacodynamics
Butyric acid (pKa 4.82) is fully ionized at physiological pH, so its anion is the material that is mainly relevant in biological systems. It is one of two primary endogenous agonists of human hydroxycarboxylic acid receptor 2 (HCA2, aka GPR109A), a Gi/o-coupled G protein-coupled receptor (GPCR), Like other short-chain fatty acids (SCFAs), butyric acid is an agonist at the free fatty acid receptors FFAR2 and FFAR3, which function as nutrient sensors that facilitate the homeostatic control of energy balance; however, among the group of SCFAs, only butyric acid is an agonist of HCA2.[4] It is also an HDAC inhibitor (specifically, HDAC1, HDAC2, HDAC3, and HDAC8), a drug that inhibits the function of histone deacetylase enzymes, thereby favoring an acetylated state of histones in cells.[4] Histone acetylation loosens the structure of chromatin by reducing the electrostatic attraction between histones and DNA.[4] In general, it is thought that transcription factors will be unable to access regions where histones are tightly associated with DNA (i.e., non-acetylated, e.g., heterochromatin). Therefore, butyric acid is thought to enhance the transcriptional activity at promoters,[4] which are typically silenced or downregulated due to histone deacetylase activity.
References
[1]Riemenschneider, Wilhelm (2002). "Carboxylic Acids, Aliphatic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a05_235.
[2]Williams, R. Scott. "Care of Plastics: Malignant plastics". WAAC Newsletter. 24 (1). Conservation OnLine. Retrieved 29 May 2017.
[3]Kasumyan A, D?ving K (2003). "Taste preferences in fishes". Fish and Fisheries. 4 (4): 289–347. doi:10.1046/j.1467-2979.2003.00121.x.
[4]Bourassa MW, Alim I, Bultman SJ, Ratan RR (June 2016). "Butyrate, neuroepigenetics and the gut microbiome: Can a high fiber diet improve brain health?". Neurosci. Lett. 625: 56–63. doi:10.1016/j.neulet.2016.02.009. PMC 4903954. PMID 26868600.
You may like
Related articles And Qustion
See also
Lastest Price from Butyric Acid manufacturers

US $10.00/kg2025-04-21
- CAS:
- 107-92-6
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 100 mt

US $278.00/KG2025-04-02
- CAS:
- 107-92-6
- Min. Order:
- 5000KG
- Purity:
- 99.5% Min
- Supply Ability:
- 1000 tons/month